EVOLVING
INNOVATION
ZETA Transcatheter Aortic Valve System
The only Balloon-Expandable valve solution designed for Aortic Regurgitation + Aortic Stenosis
Not an approved device, for investigational use only
Solving Today's Unmet Needs



AORTIC REGURGITATION
- 1 in 8 people over 75 years old have valvular heart disease (VHD)
- AR is the third most common VHD, with 4.9 million patients in 2023
Patient Risks:
- AR exponentially increases mortality
- Once symptoms arise, mortality risk > 25%
- Death often occurs within 4 years of angina
There are no approved devices in the US, patients are currently treated with Medication, Surgery, or Off-Label devices:
Medication– Only for mild AR, severe AR is not treated
Surgery– Open heart, not all patients are surgical candidates, extended hospital/recovery
Off-Label TAVR– Current devices require elevated calcification in the annulus or risk embolization; paravalvular leak risk; improper sizing causes leaks – all issues lead to heart failure
Advanced Heart Valve Replacement Technology
Combines Balloon-Expandable and Self-Expandable technologies to achieve superior heart valve performance to meet patient and physician needs.
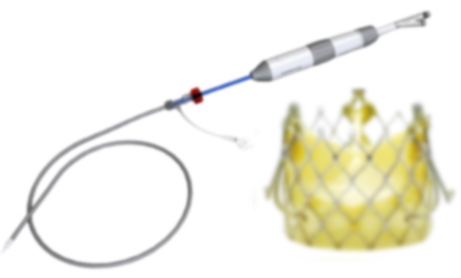
ZETA Balloon Expandable Aortic Valve
A state-of-the-art valve design that combines the proven benefits of balloon-expandable valve systems for stenosis with the addition of self-expanding valve features to treat aortic regurgitation, and is capable of treating stenosis. Designed using industry-proven materials, the Zeta Aortic Valve is designed to be simple, reliable, with accurate deployment, and includes an easy-to-use delivery system.
Benefits:
- Simple Balloon Expandable deployment for Aortic Regurgitation
- Delivery System Deflection, Rotation, and Telescoping Control for complex anatomies
- Six Atraumatic Arms for Simple Positioning- ensures proper pinning of native leaflet
- Balloon Expandable for Reduced Pacemaker Rate
- Low Valve Height for improved Coronary Clearance
- Valve Commissure alignment may improve patient results
Clinical Updates
Zeta Aortic Valve – 6 Successful Clinical Cases Completed
| CLINICAL CASE | DATE COMPLETED | FOLLOWUP | STATUS |
| 1 | September 2023 | 60d | ZETA Complete Resolution |
| 2 | December 2023 | 30d | ZETA Complete Resolution |
| 3 | December 2023 | 30d | ZETA Complete Resolution |
| 4 | December 2023 | 30d | ZETA Complete Resolution |
| 5 | December 2023 | 30d | ZETA Complete Resolution |
| 6 | December 2023 | 30d | ZETA Complete Resolution |
Patient #1 FIM, Sep 27, 2023 - ZETA Valve Deployment
Not an approved device, for investigational use only
TAVR and Aortic Regurgitation Market
Patients with Aortic Regurgitation
TAVR Market in 2023
Aortic Regurgitation market in in 2023
Annual Growth through 2027
Latest News
Laguna Tech USA Announces Site Record Number of Transcatheter Heart Valves Implanted at Single Center on Same Day in First-in-Human Clinical Trial
RVINE, Calif., Jan. 03, 2024 (GLOBE NEWSWIRE) — Laguna Tech USA, a privately-held medical technology company dedicated to innovations in structural heart solutions to broaden

Laguna Tech USA Announces Newly Designed Heart Valves with Advanced Engineering Successfully Implanted in First-in-Human Clinical Trial
IRVINE, Calif., Oct. 24, 2023 (GLOBE NEWSWIRE) — Laguna Tech USA, a privately-held medical technology company dedicated to innovations in structural heart solutions to broaden

Laguna Tech USA Begins FIH Trial of Alpha TAVR System
June 21, 2023—Laguna Tech USA announced that the first patient has been treated in the first-in-human clinical trial of the company’s investigational Alpha transcatheter aortic
